More Pediatricians Prescribe Spinosad Topical Suspension 0.9%
Spinosad Topical Suspension 0.9% is a proven safe and effective solution for resistant head lice. To date, more than 70% of pediatric specialists in the US have treated head lice with the active compound spinosad.1,5
Superiority Over Nix®
Spinosad Topical Suspension 0.9% is the only FDA-approved prescription head lice treatment that demonstrated clinical superiority to Nix® (permethrin 1%) in two head-to-head, phase III clinical trials. No nit combing required.2
In two multicenter, randomized, controlled clinical studies under actual-use conditions, significantly more patients using Spinosad Topical Suspension 0.9% were lice-free (no live lice, adults or nymphs) 14 days after the last treatment without nit combing compared to permethrin with combing, 84.6% vs. 44.9% and 86.7% vs. 42.9%, respectively (P<0.001).2
Soil microorganism at the center of the safety profile
The active ingredient in Spinosad Topical Suspension 0.9% derives from a naturally occurring soil microorganism3 that differs from neurotoxic agents such as permethrins and pyrethrins4:
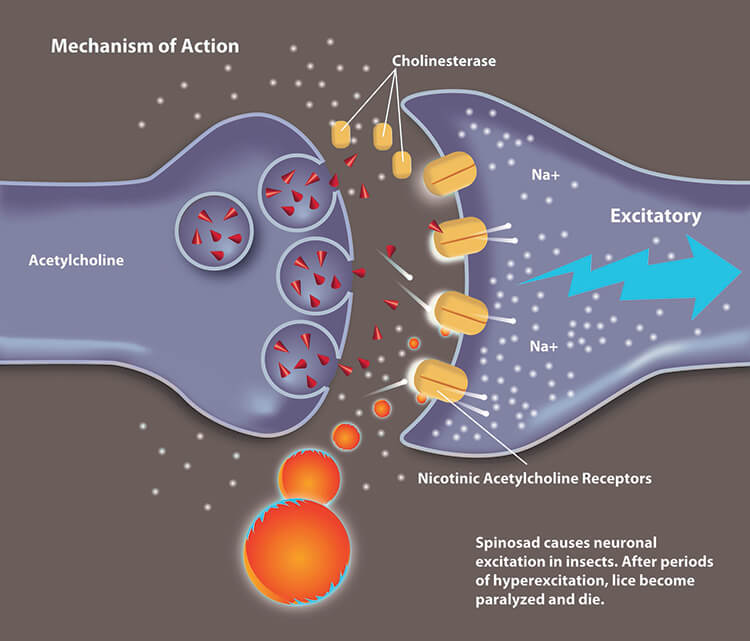
- The active compound, spinosad, is not systemically absorbed, even in patients as young as six (6) months of age5
- Spinosad targets lice and nits where they live, not penetrating beyond the stratum corneum, before sloughing off through the natural process of non-pathologic desquamation (over approximately 14 days)2
- In a Phase I clinical trial, fourteen (14) subjects 4-15 years of age, applied a single topical (scalp) treatment of spinosad 1.8% (double the active amount of compound in Spinosad Topical Suspension 0.9%) for 10 minutes. Results demonstrated no systemic absorption of spinosad2
- There is no evidence of neurotoxicity, developmental/reproductive toxicity, immunotoxicity, mutagenicity, or carcinogenicity from spinosad exposure2
Spinosad Topical Suspension 0.9% has also demonstrated similar or lower treatment-related AEs than Nix® (permethrin 1%), the leading OTC medication.6
There is no known resistance to the active compound, spinosad, in head lice and cross-resistance with other insecticides has not been reported for spinosad.7
Nix® is a registered trademark of Prestige Consumer Healthcare Inc.
Reference(s)
- IQVIA: June 11, 2021.
- Data on File
- Millar, N. S. & Denholm, I. Nicotinic acetylcholine receptors: targets for commercially important insecticides. Invert. Neurosci. 7, 53-66.
- CDC-Headlice-Treatment-OTC Medications (8.18.2016). Retrieved from https://www.cdc.gov/parasites/lice/head/treatment.html
- Spinosad Topical Suspension 0.9% Prescribing Information
- Stough D, Shellabarger S, Quiring J, Gabrielsen A. Efficacy and safety of spinosad and permethrin crème rinses for pediculosis capitis (head lice). Pediatrics. 2009;124(3): e389-395.
- Spinosad Technical Bulletin Dow AgroSciences LLC (Now Corteva agriscience), Form No. Y45-000-001 (01/01) CBK.
SPN-RRW8-002
